- What is MMR?
- What is Measles?
- Measles Symptoms
- Measles Complications
- Measles Conventional Treatment
- How Common Is Measles?
- What is Mumps?
- Mumps Symptoms
- Mumps Complications
- Mumps Conventional Treatment
- How Common Is Mumps?
- What is Rubella?
- Rubella Symptoms
- Rubella Complications
- Rubella Conventional Treatment
- How Common Is Rubella?
- When is the MMR Vaccine Given?
- Efficacy of the MMR Vaccine
- How Long Does MMR Vaccine Protection Last?
- Ingredients in the MMR Vaccine
- Controversial Ingredients In the MMR Vaccine
- MMR FDA Package Inserts
- Controversy and the MMR Vaccine
- MMR Vaccine Reactions
- FAQs
- Who Should Not Get The MMR Vaccine?
- Bottom Line and the MMR Vaccine
What is MMR?
MMR stands for Measles, Mumps, and Rubella. The MMR vaccine is a live virus vaccine intended to protect against these infections. It contains weakened forms of the viruses, which stimulate the immune system to build defenses without causing the actual diseases.
Read on to learn about measles, mumps, and rubella as well as the risks, benefits, and controversies surrounding the MMR vaccine.
What is Measles?
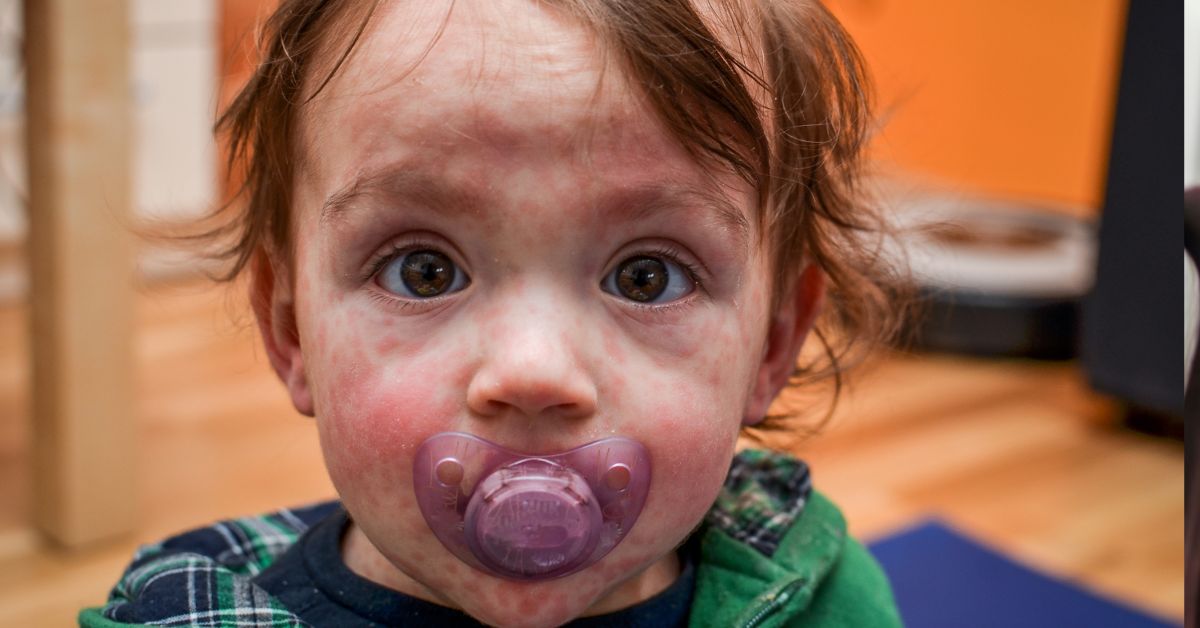
Measles is an acute systemic viral infectious disease, most likely occurring in late winter and early spring. Characteristic symptoms of fever, rash (red, round bumps and spots all over the body), red eyes, runny nose, and cough (1).
Measles Symptoms
Measles symptoms typically appear 7 to 14 days after exposure to the virus. The illness begins with early signs such as fever, cough, runny nose, and red, watery eyes. A few days later, small white spots, known as Koplik’s spots, may appear inside the mouth. The hallmark measles rash develops 3 to 5 days after the initial symptoms, starting on the face and spreading downward to the rest of the body. On light skin tones, the rash often appears as flat red spots, while on darker skin tones, it may appear as raised or flat areas that are darker than the surrounding skin. The rash is often accompanied by a high fever, which can peak at 103-105°F (39.5-40.5°C). Symptoms usually last about a week but can lead to serious complications, especially in children under 5, adults, and those with weakened immune systems (1).
Measles Rash Characteristics:
- Starts at the hairline and spreads to the face and neck.
- Over the next three days, it gradually extends downward and outward, eventually covering the hands and feet. Palms and soles are usually spared.
- The rash is made up of maculopapular spots (a mix of flat and raised lesions) that are usually separate but can merge, especially on the upper body.
- At first, the spots turn pale or white when pressed.
- After 3 to 4 days, the spots typically stop blanching under pressure.
- In severe cases, the rash may peel or flake in affected areas.
- The rash disappears in the same order it appeared, fading from the head to the extremities (1).
Measles Complications
Between 1987 and 2000, about 30% of measles cases in the United States involved at least one complication. These complications included diarrhea, ear infections, pneumonia, encephalitis, a rare condition called subacute sclerosing panencephalitis, and even death. Children under 5 years old and adults were the most likely to experience these complications (1).
Below are examples of mild, moderate, and severe cases of measles based on 1985-1992 surveillance data from the CDC:
MILD CASE
- low to mid grade fever
- rash (red, round bumps and spots all over body)- 14 days after initial exposure and starts on head and neck, lasts 5-6 days
- red eyes
- runny nose
- cough
- The child will be housebound for about a week’s time and then return to normal daily activities thereafter
MODERATE CASE
- the above symptoms accompanied with ear “infection” (7% of cases) – easily treated with antibiotics when appropriate
- pneumonia (6% of cases) – easily treated with antibiotics when appropriate
- diarrhea (8% of cases)
- a high fever – may be miserable for a few days
SEVERE CASE
- encephalitis or encephalopathy (0.1% of cases)
- seizures (0.6% of cases)
- death (0.2% of cases- 15% of the 0.1% encephalitis patients or complications with pneumonia)
Measles Conventional Treatment
There is no specific medical treatment, and the virus must run its course, then 100% lifelong immunity.
Vitamin A is recommended by the World Health Organization for children with measles in areas where vitamin A deficiency may be present as deficiency in this vitamin is a well-known risk factor for severe measles (2).
How Common Is Measles?
Measles is very rare in the United States. Before the vaccine was introduced in 1963, there were about 500,000 reported cases and 500 deaths each year, with the actual number of cases estimated to be 3 to 4 million annually. Since the vaccine became available, the incidence of measles has dropped by over 95%. However, outbreaks still occur, particularly in communities with low vaccination rates (2).
The numbers of measles from recent years according to the CDC (3):
- 2024: 284 cases
- 2023: 59 cases
- 2022: 121 cases
- 2021: 49 cases
- 2020: 13 cases
- 2019: 1274 cases
- 2018: 382 cases
- 2017: 120 cases
- 2016: 86 cases
- 2015: 191 cases
What is Mumps?
Mumps is an acute viral infectious disease that primarily affects the salivary glands, particularly the parotid glands, causing painful swelling. It is most common in late winter and spring. Typical symptoms include fever, headache, muscle aches, fatigue, and loss of appetite, often accompanied by the hallmark swelling of the cheeks and jaw (4).
Mumps Symptoms
Mumps symptoms typically develop 16 to 18 days after exposure but can appear anywhere between 12 and 25 days. Common symptoms include fever, headache, muscle aches, fatigue, loss of appetite, and swelling of the salivary glands, particularly the parotid glands, leading to puffy cheeks and a tender jaw. However, not everyone with mumps shows symptoms—prior to the introduction of vaccines, about 15-25% of infected individuals remained asymptomatic, though they could still spread the virus to others. In the vaccine era, it is unknown how many are asymptomatic (4).
Mumps Complications
Mumps can lead to complications, even without the typical swelling of the salivary glands. These complications are more common in adults than children and occur less frequently in vaccinated individuals. Serious outcomes are rare, but some of the possible complications include:
- Orchitis (inflammation of the testicles):
- Occurs in about 30% of unvaccinated and 6% of vaccinated post-pubertal males.
- Pain subsides in about a week, but tenderness may last longer.
- About half of cases result in testicular shrinkage (atrophy), though infertility is not proven to increase.
- Oophoritis and Mastitis (inflammation of the ovaries or breasts):
- Affects up to 7% (oophoritis) and 30% (mastitis) of unvaccinated post-pubertal women
- Rare in vaccinated individuals, occurring in 1% or fewer of cases.
- Other rare complications:
- Meningitis (up to 10% of unvaccinated cases): Typically mild.
- Pancreatitis (up to 4% of cases).
- Hearing loss (up to 4% of cases): Usually temporary but can be permanent.
- Severe outcomes like encephalitis, nerve damage, or death are extremely rare, particularly in the post-vaccine era (4).
Below are examples of mild, moderate, and severe cases of mumps:
MILD CASE
- (mostly seen in young children)
- fatigue
- low grade fever
- sore throat (mild)
- may not even know they have mumps
MODERATE
- (mostly seen in teens and young adults)
- high fever
- sore throat
- body aches
- fatigue
- swollen facial glands- cheeks (parotid glands)
SEVERE
- (mostly seen post pubertal)
- painful swelling of the testicles (orchitis)
- painful swelling of the ovaries (oophoritis)pancreatitis
- deafness
- Meningitis
- Encephalitis
- death – extremely rare; no reported deaths in recent mumps outbreaks
Mumps Conventional Treatment
There is no specific medical treatment, and the virus must run its course, then likely 100% lifelong immunity (4).
How Common Is Mumps?
Mumps was once very common in the United States, with around 152,000 cases reported in 1968. However, after the introduction of the mumps vaccine, cases dropped dramatically. By the mid-1980s, fewer than 3,000 cases were reported annually. Despite this, there was a resurgence of mumps in the 1980s, particularly among school-aged and college-aged individuals who had not yet been vaccinated. This led to the recommendation for a second dose of the MMR (measles, mumps, rubella) vaccine in 1989, which helped reduce mumps cases even further (4).
After the two-dose recommendation, mumps cases steadily declined until 2004. However, since 2006, the U.S. has seen an increase in mumps cases, with several large outbreaks reported, particularly in close-contact settings like schools, universities, and sports teams. Despite high vaccination coverage, many of these cases occurred in people who had received two doses of the vaccine. The majority of these outbreaks were localized, and most affected fully vaccinated individuals (4).
Number of cases of mumps per year according to the CDC (5):
- 2024: 357 cases
- 2023: 436 cases
- 2022: 386 cases
- 2021: 189 cases
- 2020: 694 cases
- 2019: 3780 cases
- 2018: 2251 cases
- 2017: 6109 cases
- 2016: 6366 cases
- 2015: 1329 cases
What is Rubella?
Rubella is a contagious viral infection, also known as German measles, that typically causes a mild illness. It is most common in late winter and spring. Symptoms often include a low-grade fever, rash, swollen lymph nodes, and joint pain, though some people may have no symptoms at all. While usually mild in children, rubella can cause serious complications during pregnancy, leading to congenital rubella syndrome in newborns (6).
Congenital Rubella Syndrome (CRS)
The primary goal of rubella vaccination programs is to prevent congenital rubella syndrome (CRS), a serious condition that occurs when a pregnant person is infected with rubella, especially in the first trimester. CRS can cause miscarriages, stillbirths, or severe birth defects in infants. These birth defects may include hearing loss, vision problems like cataracts or glaucoma, and congenital heart disease (6).
Rubella Symptoms
Rubella symptoms usually develop 14 to 17 days after exposure, though they can appear between 12 and 23 days. A characteristic sign is a mild rash that begins on the face and spreads downward to the body. On lighter skin, the rash appears as fine pink spots, while on darker skin, it may look like subtle raised areas or darker patches. Other symptoms include low-grade fever, swollen lymph nodes, headache, and joint pain, especially in adults. However, up to 50% of people infected with rubella may have no symptoms but can still spread the virus to others (6).
Rubella Complications
While rubella is generally mild, complications can occur in rare cases.
- Encephalitis (inflammation of the brain) 1 in 6,000 cases
- Bleeding issues caused by low platelet levels and damage to blood vessels which can lead to easy bruising or bleeding in areas like the skin, gi tract, brain, or kidneys 1 in 3,000 cases
- Other even more rare complications: orchitis (testicular inflammation), neuritis (nerve inflammation), progressive panencephalitis (neurological disorder) (6).
Below are examples of mild, moderate, and severe rubella cases:
MILD
- mild fever
- generic rash that goes unnoticed
MODERATE
- body and joint aches
- rash
- fever
SEVERE
- Congenital Rubella Syndrome (CRS)- birth defects (deafness, cataracts, heart defects, liver and spleen damage, brain damage, and possibly stillbirth), risk is in first trimester, somewhat second trimester, and no risk in third trimester.
Rubella Conventional Treatment
There is no specific medical treatment, and the virus must run its course, then 100% lifelong immunity.
How Common Is Rubella?
Rubella has become very rare in the United States thanks to widespread vaccination efforts. Since the introduction of the vaccine in 1969, cases of rubella and congenital rubella syndrome (CRS) have declined dramatically. In 2004, endemic rubella was declared eliminated in the U.S., with fewer than 10 cases of rubella and less than one CRS case reported annually. Since 2012, all rubella cases in the U.S. have been linked to infections acquired outside the country (6).
When is the MMR Vaccine Given?
Age 12 months with booster dose at 5 yrs.
For more information: CDC/Alternative Vaccine Schedules
Efficacy of the MMR Vaccine
The MMR vaccine has quite good efficacy overall. When reading the statistics, keep in mind that when a vaccine is 95% effective, that means that 95% of children are protected, not that each individual child is protected 95% worth.
Below we list both the percentage of children that develop antibodies and the overall effectiveness, which indicates what percentage of children are actually protected.
Measles
- 1 dose: 95% of children develop measles antibodies
- 2 doses: 99% of children develop measles antibodies
- Though the titres of antibodies from vaccines are lower than from a natural infection, it seems they provide full protection. Immunity is long-term and probably lifelong (1).
Mumps
- 1 dose: 94% of children develop mumps antibodies
- Post licensure studies show that the vaccine effectiveness is 78% after one dose and 88% after 2 doses (4).
Rubella
- 1 dose: 95% of children develop antibodies to rubella; 90% are immune for 15+ years
- Long-term protection: A single dose provides long-term, likely lifelong, protection.
- While antibody levels may decline over time, this does not appear to increase the risk of clinical rubella or congenital rubella syndrome (CRS). Vaccinated individuals are highly protected, and CRS cases in vaccinated people are extremely rare in the United States.
Because the efficacy of one dose of MMR vaccine is so high, sometimes parents choose to forego the second dose. In places with vaccine mandates, children usually need two doses of the MMR vaccine. However, if blood tests (titers) show they’re immune to measles, mumps, and rubella, some areas may accept this instead of more doses. Rules vary, so it’s best to check with local health authorities or schools.
How Long Does MMR Vaccine Protection Last?
Although the titer of vaccine-induced antibodies is lower than that following natural disease, both serologic and epidemiologic evidence indicate that vaccine-induced immunity appears to be long-term and probably lifelong in most persons.
Ingredients in the MMR Vaccine
M-M-R-II
- Active Components: Measles, mumps, and rubella virus strains (weakened)
- Stabilizers: Sorbitol, sucrose, hydrolyzed gelatin, recombinant human albumin
- Residual Traces: Neomycin, fetal bovine serum, and traces of cell culture proteins
PRIORIX
- Active Components: Measles, mumps, and rubella virus strains (weakened)
- Stabilizers: Lactose, sorbitol, amino acids, and mannitol
- Residual Traces: Neomycin (antibiotic), egg protein, and bovine serum
- Diluent: Sterile water
ProQuad
- Active Components: Measles, mumps, rubella, and chickenpox virus strains (weakened)
- Stabilizers: Sucrose, Sorbitol, Hydrolyzed gelatin, Urea, Sodium chloride, Sodium phosphate, Monosodium L-glutamate, Recombinant human albumin, Sodium bicarbonate, Potassium phosphate, Potassium chloride
- Residual Traces: MRC-5 cell proteins and DNA, Neomycin, Bovine calf serum
Controversial Ingredients In the MMR Vaccine
The following ingredients in MMR (Measles, Mumps, Rubella) and MMRV (Measles, Mumps, Rubella, Varicella) vaccines have been associated with some controversy due to ethical, health, or religious concerns:
- Fetal Bovine Serum (FBS): Used in the cell culture process for virus propagation, including for the production of the mumps and rubella components. Some individuals have concerns due to its animal origin.
- Human Cell Lines (WI-38, MRC-5, and others): These are cell lines derived from aborted human fetuses, which are used in the production of some vaccines, including rubella (WI-38) and varicella (MRC-5). Some religious and ethical groups object to their use based on the origin of the cell lines.
- Rubella Virus (Wistar RA 27/3 strain): Derived from a rubella-infected fetus aborted in the 1960s. This raises ethical concerns for those opposed to abortion.
- Neomycin: An antibiotic used in the vaccine preparation process. Some individuals may have allergic reactions or concerns about antibiotic residues in vaccines.
- Gelatin (Hydrolyzed Gelatin): Derived from animal collagen (typically from pigs or cows), gelatin is used as a stabilizer in vaccines. It may be a concern for individuals with religious or dietary restrictions, such as those who follow kosher or halal diets.
- Egg Protein: Egg protein may be present as a residual trace due to the use of chicken embryos in the virus propagation process. This is a concern for individuals with egg allergies.
Learn more about the individual vaccine ingredients here: Vaccine Ingredients
MMR FDA Package Inserts
- M-M-R II (Merck) FDA Package Insert
- PRIORIX (GlaxoSmithKline) FDA Package Insert
- ProQuad (fridge) FDA Package Insert
- ProQuad (frozen) FDA Package Insert
Controversy and the MMR Vaccine
- Atypical Measles from Killed Measles Virus Vaccine (No Longer in Use)
- Autism Connection & Lack of Safety Data
- Aborted Fetal Cells & Rubella from Aborted Fetus
Atypical Measles From Killed Measles Virus Vaccine (No Longer In Use)
The killed measles virus vaccine, used between 1963 and 1967, was an early attempt to prevent measles. Unlike the live attenuated vaccine, it used inactivated virus particles, which induced incomplete immunity. This partial immune response led to the development of “atypical measles” in some vaccinated individuals when they were later exposed to the live virus. Atypical measles caused severe symptoms, including high fever, intense headache, and a distinctive rash, often accompanied by pneumonia or pleuritis (7).
The killed vaccine was discontinued in 1967, replaced by the safer and more effective live attenuated vaccine. Individuals vaccinated with the killed version were advised to be revaccinated to ensure full protection. This episode highlights the importance of understanding immune responses in vaccine development (7).
The Possible Association Of Autism With MMR & Lack Of Safety Data Overall
In 1998, Andrew Wakefield published a case series suggesting a possible connection between developmental disorders, including autism, and the MMR vaccine. This sparked widespread debate and a lasting controversy that continues more than 25 years later (8). It also prompted decades of extensive research into vaccine safety, particularly for the MMR vaccine.
An earlier version of this article referenced a Cochrane Review, which once noted limitations in the design and reporting of MMR vaccine safety studies (9). Since then, significant progress has been made, with the latest review analyzing 138 studies involving over 23 million participants. According to this updated review, there is no evidence linking the MMR vaccine to encephalitis, encephalopathy, or autism spectrum disorders (1).
The same study found there’s no evidence linking MMR vaccines to other health concerns like asthma, diabetes, or cognitive delays. There wasn’t enough evidence to assess the risk of inflammatory bowel disease (1).
Risks identified by the study: increased risk of febrile seizure – 1 per 1700 to 1 per 1150 administered doses, and increased risk of idiopathic thrombocytopenia purpura (a rare blood disorder) – 1 per 40,000 administered MMR doses (1).
You can read the full review here: Vaccines for measles, mumps, rubella, and varicella in children
Vaccines Manufactured With Aborted Fetal & Embryonic Components
Some vaccines, including the MMR vaccine, are produced using cell lines derived from fetal tissue obtained from elective terminations in the 1960s. For example, the rubella component of the MMR vaccine relies on such cell lines because the rubella virus used in its development originated from a fetus affected by the disease. These cell lines have been maintained in laboratories for decades and are not the same as fresh tissue. While no fetal tissue is present in the final vaccine, this process raises ethical questions for individuals across various religious and moral traditions. Many religious authorities have issued guidance affirming the permissibility of using these vaccines when they protect against serious diseases, as the preservation of life is a core principle many faiths.
This is a decision that each family must make individually. We recommend discussing it with your faith community and leaders if you need clarity on your religious guidelines.
MMR Vaccine Reactions
SIGNS TO LOOK FOR:
- fever
- mild rash in 2-5 percent
- adult women have a 12 to 26 percent rate of transient arthritis symptoms that can range from mild to severe
- fainting
- headache
- dizziness
- body aches
- irritability
- vomiting
- diarrhea
- parotid gland swelling (in the cheeks)
- lymph node swelling
KNOWN SEVERE REACTIONS:
- panniculitis (tender and inflamed skin nodules, sometimes with fatigue and weight loss)
- measles infection
- vasculitis (inflammation of the blood vessels)
- inflammation of the pancreas
- diabetes
- thrombocytopenia (an autoimmune reaction in which the platelet cells in the blood decrease, causing bleeding problems; occurs in 1 in 30,000 doses)
- elevation of white blood cell counts
- severe anaphylaxis (less than 1 in 1 million doses)
- less severe allergic reactions such as swelling or hives, muscle inflammation, pneumonia or pneumonitis (nonbacterial inflammation in the lungs)
- Stevens-Johnson syndrome (a severe allergic reaction involving the skin and some internal organs)
- nerve deafness in the ear
- severe eye inflammation that can permanently affect vision
- testicular pain and swelling
KNOWN NEUROLOGICAL REACTIONS:
- encephalitis in 1 in 1 million doses (inflammation and swelling within the brain; symptoms include high fever, severe headache, and inconsolable crying)
- encephalopathy (damage and dysfunction of the brain as a rare result of encephalitis)
- subacute sclerosing panencephalitis (SSPE, a type of gradual-onset encephalitis that eventually results in encephalopathy over several years)
- Guillain-Barré syndrome (a temporary muscle weakness or paralysis)
- seizures with or without fever (1 in 3000 doses)
- ataxia (balance problems with difficulty walking)
- multiple nerve inflammation and dysfunction
- aseptic meningitis (likely triggered by one of the vaccine viruses)
- measles inclusion body encephalitis (MIBE)
POSTMARKETING SURVEILLANCE:
Adverse reactions that have been reported by the public since the introduction of the vaccine:
- subacute sclerosing panencephalitis (SSPE, a type of gradual-onset encephalitis that eventually results in encephalopathy over several years)
- aseptic meningitis
- encephalitis
- encephalopathy
- Respiratory System: Pneumonia, pneumonitis, sore throat, cough, rhinitis
- Skin: Stevens-Johnson syndrome, erythema multiforme, urticaria, rash, measles-like rash, pruritus.
- Local reactions, including burning/stinging at injection site, wheal and flare, redness (erythema), swelling, induration, tenderness, vesiculation at injection site
- Special Senses: Ear- nerve deafness, otitis media
- Special Senses: Eye- retinitis, optic neuritis, papillitis, retrobulbar neuritis, conjunctivitis
- Urogenital System: epididymitis, orchitis
- Death from various, and in some cases unknown, causes has been reported rarely following vaccination with measles, mumps, and rubella vaccines; however, a causal relationship has not been established in healthy individuals.
FAQs
1. Does This Vaccine Protect The Community?
Yes, different components help protect the community in different ways.
Measles: The MMR vaccine helps protect vulnerable community members from measles through herd immunity (11). This is particularly true for children in schools and daycares where they are around their peers for a large part of the day in classrooms and where measles could easily transmit.
Mumps: In theory the MMR vaccine should contribute to herd immunity from mumps; however, waning immunity in adults and outbreaks of mumps amongst vaccinated people bring this into question (12-14).
Rubella: This aspect of the vaccine helps prevent the spread of rubella to pregnant women in the child’s family or community. This is important because contracting rubella while pregnant can lead to fetal death or permanent disability.
2. Does This Vaccine Shed Or Cause Infection?
Yes, MMR is a live virus vaccine so it has the potential to shed. However, it isn’t likely to be transmissible (15). When it comes to the MMR vaccine, there has only been one documented case of possible measles transmission: a sibling contracted measles from a recently vaccinated sibling in 1989 – this was never confirmed with lab testing (16).
There have been no reported cases of mumps that have arisen from vaccination. There have been some reports of rubella passing through breast milk – in most cases the virus is detected in breast milk or through infant antibodies; there has been one case in which an infant contracted rubella (potentially through breast milk exposure) – they recovered fully (17).
Given that there have been millions of MMR vaccine doses given and only two unconfirmed cases where the vaccine strain may have been transferred, I would say that the risk of infection from a recently vaccinated person is astronomically low. A 2016 meta-analysis looking specifically at the measles portion of the vaccine concluded that there is no evidence of human-to-human transmission of vaccine-derived measles (15).
Pregnant mothers wishing to be extremely cautious could wait to vaccinate their older children until they are in their third trimester and the risk of congenital rubella syndrome (CRS) has fully passed. To be very clear, this is theoretical only: there have been no reports of rubella passing from a vaccinated child to their mother or of the subsequent development of CRS.
Allowing unvaccinated children to play with children recently vaccinated with MMR doesn’t present a danger to the unvaccinated children.
All this being said, there is still a theoretical risk of transmission, particularly for immunocompromised people. If you, or a family member, falls into this category, consult your doctor for guidance.
3. Is It Better To Get MMR Or MMR-V?
MMR-V has twice the risk of febrile seizure following vaccination compared to MMR.* Anyone with a personal or family history of seizure is recommended to receive the MMR instead of MMR-V vaccine (1,18).
MMR and varicella vaccines may be given in the same visit at separate sites (e.g. in separate arms). This strategy reduces the effect of extra adverse events (18).
*Risk of febrile seizure following MMR-V is 8 out of 10,000; risk of separate MMR and varicella vaccines given in the same visit is 4 out of 10,000 (18).
4. Can I Get These Vaccines Separately, Or Do I Have To Get MMR Together?
At present there are no available individual vaccines, only combination MMR and MMR-V. See package inserts for more information.
5. How Can I Reduce The Number Of Doses Needed?
Policies about MMR (measles, mumps, rubella) vaccination requirements vary depending on local or national regulations. In many places where vaccines are mandated for school or childcare attendance, children are typically required to have two doses of the MMR vaccine. However, if a child has documented immunity to all three diseases (measles, mumps, and rubella) through serologic testing (titers), they may be exempt from additional doses in some jurisdictions.
This approach is generally acceptable in places where vaccine mandates allow for proof of immunity as an alternative to vaccination. It is important to confirm specific requirements with the relevant health authority or school system, as policies can differ. Some institutions may still require vaccination regardless of titer results for administrative simplicity or to ensure consistent protection.
Who Should Not Get The MMR Vaccine?
Because the MMR vaccine is a live virus vaccine, there are more precautions and contraindications than with inactivated vaccines like DTaP, Hib, etc. The following is a list of contraindications – people who shouldn’t get the vaccine – and precautions – people who should wait or take extra care when getting the vaccine.
They revolve around allergy, immune compromise, increased bleeding risk, seizures, and drugs that can kill the live viruses in the vaccines before they can instigate a protective immune response.
From the CDC Pink Book on Measles:
MMR Vaccine Contraindications (1)
- Severe allergic reaction to vaccine component or following a prior dose
- Severe immunocompromise
- Systemic high-dose corticosteroid therapy for 14 days or more
- HIV infection, regardless of immunocompetence status*
- Family history of congenital or heredity immunodeficiency in first-degree relatives
- Pregnancy
*MMRV only
MMR Vaccine Precautions (1)
- Moderate or severe acute illness
- Alpha-gal allergy (consult with physician)
- Receipt of antibody-containing blood products (wait 3 to 11 months to vaccinate)
- History of thrombocytopenic purpura or thrombocytopenia
- Need for tuberculin skin testing or interferon-gamma release assay testing
- Simultaneous use of aspirin or aspirin-containing products*
- Personal or family history of seizures of any etiology*
- Receipt of specific antiviral drugs 24 hours before vaccination*
*MMRV only
Bottom Line and the MMR Vaccine
What does MMR protect against? MMR protects against measles, mumps, and rubella.
How common are measles, mumps, and rubella? These infections are uncommon in the United States, though there are regular outbreaks for measles and mumps. Measles outbreaks occur mostly in under-vaccinated communities (19) while mumps outbreaks occur mostly in vaccinated communities. There are less than 10 reported cases of rubella per year – most are imported from other countries.
What is the benefit of vaccinating my child with MMR? The main benefit of vaccinating your child is direct protection against these diseases in the event of a local outbreak or when traveling to an area of the world where the disease is more common. You also contribute to herd immunity against these diseases, which seems to be an effective strategy when it comes to measles and rubella, but less so when it comes to mumps. Finally, you reduce the risk of your child passing rubella to a pregnant family or community member, which could result in miscarriage or congenital rubella syndrome. If you have a daughter, vaccinating her with MMR means that when she reaches child-bearing age she will likely be protected against rubella.
What are the potential risks of vaccinating with MMR? The MMR vaccine carries a higher list of side effects and precautions compared to most other childhood vaccines because it is a live virus vaccine. Be sure to read the package inserts for a full understanding of risks and benefits.
Reference:
- Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, Kroger A, Wolfe S, eds. 14th ed. Washington D.C. Public Health Foundation, 2021. Chapter 13: Measles. https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-13-measles.html
- D’Souza, R. M., & D’Souza, R. (2002). Vitamin A for treating measles in children. The Cochrane database of systematic reviews, (1), CD001479. https://doi.org/10.1002/14651858.CD001479
- Centers for Disease Control and Prevention. (2024). Measles Cases and Outbreaks. Www.cdc.gov. https://www.cdc.gov/measles/data-research/index.html
- Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, Kroger A, Wolfe S, eds. 14th ed. Washington D.C. Public Health Foundation, 2021. Chapter 15: Mumps. https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-15-mumps.html
- Centers for Disease Control and Prevention. (2025). Mumps Cases and Outbreaks. Www.cdc.gov. https://www.cdc.gov/mumps/outbreaks/index.html
- Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, Kroger A, Wolfe S, eds. 14th ed. Washington D.C. Public Health Foundation, 2021. Chapter 20: Rubella. https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-20-rubella.html
- Hendriks, J., & Blume, S. (2013). Measles vaccination before the measles-mumps-rubella vaccine. American journal of public health, 103(8), 1393–1401. https://doi.org/10.2105/AJPH.2012.301075
- Wakefield, A. J., Murch, S. H., Anthony, A., Linnell, J., Casson, D. M., Malik, M., Berelowitz, M., Dhillon, A. P., Thomson, M. A., Harvey, P., Valentine, A., Davies, S. E., & Walker-Smith, J. A. (1998). Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet (London, England), 351(9103), 637–641. https://doi.org/10.1016/s0140-6736(97)11096-0 (Retraction published Lancet. 2004 Mar 6;363(9411):750. doi: 10.1016/S0140-6736(04)15715-2 Lancet. 2010 Feb 6;375(9713):445. doi: 10.1016/S0140-6736(10)60175-4)
- Demicheli, V., Jefferson, T., Rivetti, A., & Price, D. (2005). Vaccines for measles, mumps and rubella in children. The Cochrane database of systematic reviews, (4), CD004407. https://doi.org/10.1002/14651858.CD004407.pub2
- Di Pietrantonj C, Rivetti A, Marchione P, Debalini MG, Demicheli V. Vaccines for measles, mumps, rubella, and varicella in children. Cochrane Database of Systematic Reviews 2021, Issue 11. Art. No.: CD004407. DOI: 10.1002/14651858.CD004407.pub5. Accessed 27 January 2025.
- Glasser, J. W., Feng, Z., Omer, S. B., Smith, P. J., & Rodewald, L. E. (2016). The effect of heterogeneity in uptake of the measles, mumps, and rubella vaccine on the potential for outbreaks of measles: a modelling study. The Lancet. Infectious diseases, 16(5), 599–605. https://doi.org/10.1016/S1473-3099(16)00004-9
- Lewis, P. E., Burnett, D. G., Costello, A. A., Olsen, C. H., Tchandja, J. N., & Webber, B. J. (2015). Measles, Mumps, and Rubella Titers in Air Force Recruits: Below Herd Immunity Thresholds?. American journal of preventive medicine, 49(5), 757–760. https://doi.org/10.1016/j.amepre.2015.04.019
- Rasheed, M. A. U., Hickman, C. J., McGrew, M., Sowers, S. B., Mercader, S., Hopkins, A., Grimes, V., Yu, T., Wrammert, J., Mulligan, M. J., Bellini, W. J., Rota, P. A., Orenstein, W. A., Ahmed, R., & Edupuganti, S. (2019). Decreased humoral immunity to mumps in young adults immunized with MMR vaccine in childhood. Proceedings of the National Academy of Sciences of the United States of America, 116(38), 19071–19076. https://doi.org/10.1073/pnas.1905570116
- Connell, A. R., Connell, J., Leahy, T. R., & Hassan, J. (2020). Mumps Outbreaks in Vaccinated Populations-Is It Time to Re-assess the Clinical Efficacy of Vaccines?. Frontiers in immunology, 11, 2089. https://doi.org/10.3389/fimmu.2020.02089
- Greenwood, K. P., Hafiz, R., Ware, R. S., & Lambert, S. B. (2016). A systematic review of human-to-human transmission of measles vaccine virus. Vaccine, 34(23), 2531-2536.
- Millson DS. Brother-to-sister transmission of measles after measles, mumps, and rubella immunisation. Lancet. 1989 Feb 4;1(8632):271. doi: 10.1016/s0140-6736(89)91274-9. PMID: 2563426.
- Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-. Measles-Mumps-Rubella Vaccine. [Updated 2024 Aug 15]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK501034/
- Centers for Disease Control and Prevention. (2021). Q&As About Vaccination Options for Preventing Measles, Mumps, Rubella, and Varicella. www.cdc.gov. https://www.cdc.gov/vaccines/vpd/mmr/hcp/vacopt-faqs-hcp.html#
- Quinn, S. C., Jamison, A. M., & Freimuth, V. S. (2020). Measles outbreaks and public attitudes towards vaccine exemptions: some cautions and strategies for addressing vaccine hesitancy. Human vaccines & immunotherapeutics, 16(5), 1050–1054. https://doi.org/10.1080/21645515.2019.1646578
Reviewed/Updated: 01/25
Content Created: 07/14