- What is Varicella (Chickenpox)?
- Varicella Symptoms
- Incubation Period
- Communicable Period
- Conventional Treatment for Varicella
- Post Exposure Prophylaxis for Varicella
- When is the Varicella Vaccine Given?
- Dr. Green Mom’s Notes About Varicella Vaccination
- Efficacy of the Varicella Vaccine
- How Long Does Varicella Vaccine Protection Last?
- Breakthrough Varicella
- Ingredients in the Varicella Vaccines
- Varicella FDA Package Inserts
- Controversy & Confusion About the Varicella Vaccine
- Varicella Vaccine Reactions
- Who Should Not Get The Varicella Vaccine?
- The Bottom Line and the Varicella Vaccine
What is Varicella (Chickenpox)?
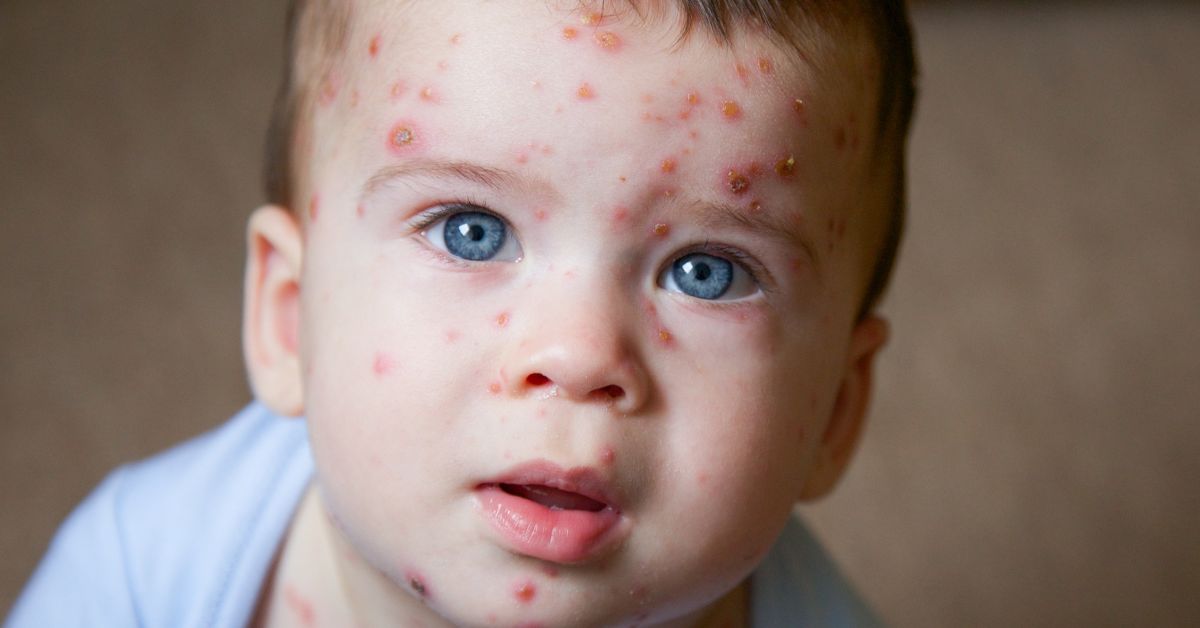
Varicella, commonly known as chickenpox, is a highly contagious viral infection caused by the varicella-zoster virus. It spreads through respiratory droplets or direct contact with the blisters of an infected person. Symptoms include an itchy rash that usually begins on the face, scalp, or trunk and spreads throughout the body; fever; fatigue; and loss of appetite. While chickenpox is most common in children, adults can contract the virus. Chickenpox in healthy children is usually mild; however, for adults, immune-compromised children, and those with HIV it can be more severe, leading to complications like pneumonia or encephalitis (1).
Once a person recovers from chickenpox, the varicella-zoster virus remains dormant in the body and can reactivate later in life causing shingles. Shingles is characterized by a painful, blistering rash, often on one side of the body, and it can cause long-lasting nerve pain.
Pregnant women who contract chickenpox are at higher risk for complications, including pneumonia, and the virus can be harmful to the fetus, potentially leading to birth defects, low birth weight, or premature birth. For these reasons, pregnant women who have not had chickenpox or been vaccinated are advised to avoid exposure (1).
There is no specific antiviral treatment for chickenpox in healthy individuals, and most cases are managed with rest, fluids, and over-the-counter medications (e.g. acetaminophen and calamine lotion) to alleviate symptoms such as fever and itching. For high-risk individuals, such as adults, pregnant women, or those with weakened immune systems, antiviral medications like acyclovir or immune globulin therapy (VariZIG) may be prescribed to reduce the severity and duration of the illness. Vaccination against varicella has significantly reduced the incidence of chickenpox, preventing many of the severe complications associated with the disease.
Varicella Symptoms
Chickenpox usually begins with a rash, which is typically the first sign in children. However, in teenagers and adults, a mild fever may appear first, followed by a rash. Symptoms usually develop 14-16 days (with a range of 10-21 days) after exposure to the varicella virus.
The rash is often itchy and generally starts on the head before spreading to the trunk, where it becomes most concentrated. As the rash progresses, new crops of pox bumps appear, so different stages of the rash—such as red bumps, blisters, and crusts—will be visible at the same time.
The lesions are usually 1-4 mm in diameter and have a characteristic appearance. They start as delicate, clear fluid-filled vesicles on an erythematous (reddened) base. As the infection progresses, these vesicles may rupture and become purulent (pus-filled) before eventually drying out and forming crusts. In addition to the skin, lesions can also occur on mucous membranes, such as in the mouth, respiratory tract, vagina, conjunctiva, and even the cornea of the eye.
The following are examples of mild, moderate, and severe cases of chickenpox (1):
Mild
- Fatigue/malaise
- Rash & pruritus (itching)
- Low grade fever 102°F (2-3 days)
Moderate
- Adults and teens over 15 years old may more intensely suffer the symptoms listed above.
Severe
- Children and adults with lymphoma, leukemia, and other immune issues may develop a severe progressive form of varicella characterized by high fever, extensive vesicular eruption, and high complication rates.
- People infected with human immunodeficiency virus (HIV) may have severe, prolonged illness.
Complications
- Secondary bacterial infection of skin lesions by resistant staph
- Pneumonia (bacterial/viral)
- Encephalitis
- Reye’s Syndrome
- Hospitalizations (1): healthy children: 1-2 per 1,000; adults: 14 per 1,000
- Death (1): ages 1-14: 1 per 100,000 cases; ages 15-19: 6 per 100,000 cases; adults: 21 per 100,000 cases
Groups at Increased Risk for Varicella Complications:
- Persons older than 15 years
- Infants younger than 1 year
- Immunocompromised
- Pregnant women
- Newborns of women with rash onset within 5 days before to 2 days after delivery
Incubation Period
The incubation period is typically 14 to 16 days after exposure but can range from 10 to 21 days. You have no signs or symptoms of chickenpox during this time. The incubation period may be longer (up to 28 days or more) for those who receive postexposure prophylaxis with varicella specific immune globulin (1).
Communicable Period
The period of communicability – when a person with chickenpox can spread the virus to others – extends from 1 to 2 days before the onset of rash until all lesions have formed crusts. Those who are vaccinated and contract varicella may develop lesions that do not crust. Isolation guidance is to restrict contact with others until no new lesions appear within a 24-hour period (1).
Varicella is highly contagious. Secondary attack rates – the spread of disease in a family, household, or dwelling unit – of varicella are between 61% and 100% (1).
Conventional Treatment for Varicella
There is no specific antiviral treatment for chickenpox in healthy individuals, and most cases are managed with rest, fluids, and over-the-counter medications to alleviate symptoms like fever and itching. Common treatments for mild cases include:
- Acetaminophen for fever relief
- Calamine lotion to soothe itching
- Plenty of fluids and rest to support recovery
For individuals at higher risk, such as adults, pregnant women, or those with weakened immune systems, antiviral medications may be prescribed to reduce the severity and duration of the illness. These treatments include:
- Acyclovir or valacyclovir (an antiviral medications) (2,3)
- Immune globulin therapy (VariZIG) (4)
- FDA information on VariZIG™
- CDC Investigational Information – VariZIG™
Post Exposure Prophylaxis for Varicella
Post-exposure prophylaxis (treatment to prevent the development of disease after exposure to the virus) with the varicella vaccine can prevent or lessen illness if given within 3 to 5 days of exposure. Studies show it is 70% to 100% effective and can be given to those over 12 months old without immunity.
If exposure does not cause chicken pox infection, the vaccine provides future protection.
In outbreaks, such as in schools, a second vaccine dose is recommended for those who previously received one to help control the spread provided that the appropriate vaccination spacing is observed (1):
- 12 months to 12 years: 3 months required between doses
- 13+ years: 4 weeks required between doses
Post-exposure prophylaxis is also possible with an immune globulin called VariZIG. VariZIG is an injection used to prevent severe chickenpox in people who can’t receive the varicella vaccine and don’t have immunity. It is made from donated plasma with high levels of varicella antibodies (IgG) and is given as an intramuscular shot.
The CDC recommends VariZIG as post-exposure treatment for (1):
- People with weakened immune systems who haven’t had chickenpox or the vaccine.
- Newborns whose mothers develop chickenpox within 5 days before and 2 days after delivery.
- Hospitalized preterm babies (born at 28+ weeks) whose mothers aren’t immune.
- Very premature babies (born before 28 weeks or under 1,000g (2.2 lbs.)), regardless of the mother’s immunity.
- Pregnant people without immunity to chickenpox.
VariZIG helps lower the risk of severe complications after exposure to the virus. If you fall into one of these groups, talk to your doctor as soon as possible after exposure.
When is the Varicella Vaccine Given?
According to the CDC schedule, the vaccine is given in two doses; one between 12-15 months and the second between 4-6 years.
For more information, see: CDC/ Alternative Vaccine Schedules
Dr. Green Mom’s Notes About Varicella Vaccination
The varicella vaccine may be given at the same visit as the MMR vaccine. However, you may wish to give the varicella vaccine and MMR vaccine at separate injection sites to reduce the risk of febrile seizure as an adverse event. If MMR and varicella vaccines are not given during the same visit, then there must be at least 4 weeks between shots for optimal efficacy (1).
Some people choose to postpone this vaccine until the teen years as this is when chickenpox increases in severity. You can test your child’s titers before vaccinating: they may have been exposed to chickenpox and built up the antibodies without knowing.
Additionally, one dose of the vaccine seems to be very effective at preventing severe disease, but breakthrough infections are possible – read more about breakthrough varicella below. Two doses are more effective at preventing chickenpox altogether. You may wish to speak to your doctor about titer testing if planning to only do one shot (5).
Efficacy of the Varicella Vaccine
One Dose
- 1 dose of single-antigen varicella vaccine is (5) —
- 82% effective at preventing any form of varicella
- Almost 100% effective against severe varicella
Two Doses
- In a pre-licensure clinical trial, 2 doses of vaccine were (5) —
- 98% effective at preventing any form of varicella
- 100% effective against severe varicella
- In post-licensure studies, 2 doses of vaccine were (5) —
- 88% to 98% effective at preventing all varicella
How Long Does Varicella Vaccine Protection Last?
We don’t know exactly how long the varicella (chickenpox) vaccine protects someone, but live vaccines usually provide long-lasting immunity.
Studies have found that people who got the varicella vaccine still had antibodies 10 to 20 years later. However, these studies were done when natural chickenpox infections were still common, so the results might not fully reflect today’s situation.
A study done from 1997 to 2003 found that one dose of the varicella vaccine was (5):
- 97% effective in the first year
- 86% effective in the second year
- 81% to 86% effective from years 2 to 8
Most children who got the vaccine and later caught chickenpox had only a mild case.
Another study showed that kids who received two doses of the vaccine were still protected 10 years later. They were also less likely to get a mild case compared to those who only had one dose. Importantly, the risk of getting chickenpox after vaccination did not increase over time (5).
Breakthrough Varicella
Breakthrough varicella refers to cases of chickenpox that occur in people who have already been vaccinated against the virus, typically more than 42 days after receiving their vaccine (6).
Breakthrough varicella can happen after one or two doses of the vaccine. The symptoms tend to be milder than in unvaccinated individuals, with fewer skin lesions, a shorter duration of illness, and less fever. However, in some extremely rare cases, especially in those who only received one dose of the vaccine, the illness can be more severe, with complications like hospitalizations or, in very rare instances, death (6).
Factors that may affect the risk for breakthrough varicella (7):
- Number of doses: two doses of a varicella vaccine are more effective than one
- Amount of time between doses: three to four years between doses seems to be the most effective
- Amount of exposure (being exposed to a handful of infected classmates vs. a whole classroom full of infected classmates): less exposure means less risk.
Overall, the vaccine helps prevent severe cases of chickenpox, but breakthrough infections can still occur (1).
Ingredients in the Varicella Vaccines
VARIVAX (Varicella Virus Vaccine Live)
- Oka/Merck strain of live, attenuated varicella virus
- Sucrose (17 mg)
- Hydrolyzed gelatin (8.3 mg)
- Urea (3.5 mg)
- Sodium chloride (2.1 mg)
- Monosodium L-glutamate (0.33 mg)
- Sodium phosphate dibasic (0.30 mg)
- Potassium phosphate monobasic (53 mcg)
- Potassium chloride (53 mcg)
- Residual Manufacturing Components: MRC-5 human diploid cell components (DNA and protein), trace amounts of neomycin, trace amounts of bovine calf serum from MRC-5 culture media
ProQuad (Measles, Mumps, Rubella, and Varicella Virus Vaccine Live)
- Measles virus (Enders’ attenuated Edmonston strain)
- Mumps virus (Jeryl Lynn™ strain)
- Rubella virus (Wistar RA 27/3 strain)
- Varicella virus (Oka/Merck strain)
- Sucrose (20 mg)
- Sorbitol (16 mg)
- Hydrolyzed gelatin (11 mg)
- Urea (2.5 mg)
- Sodium chloride (2.3 mg)
- Sodium phosphate (1.4 mg)
- Monosodium L-glutamate (0.38 mg)
- Recombinant human albumin (0.25 mg)
- Sodium bicarbonate (0.13 mg)
- Potassium phosphate (94 mcg)
- Potassium chloride (58 mcg)
- Residual Manufacturing Components: MRC-5 human diploid cell components (DNA and protein), ≤5 mcg of neomycin, ≤0.5 mcg of bovine calf serum, other buffer and media ingredients
Controversial Ingredients Explained
- MRC-5 Human Diploid Cells (DNA and Protein) – Derived from lung cells of a fetus aborted in 1966. While no new fetal tissue is used in vaccine production today, this remains an ethical concern for some individuals.
- Rubella Virus (Wistar RA 27/3 Strain) – Developed from a fetus aborted in 1964 after the mother contracted rubella. Ethical concerns exist, though no new fetal tissue is used.
- Porcine-Derived Hydrolyzed Gelatin – Used as a stabilizer but may be a concern for individuals with allergies or religious/ethical dietary restrictions.
- Neomycin – An antibiotic that can cause allergic reactions in some people.
- Bovine Calf Serum – Animal-derived ingredient, which may be concerning for those avoiding animal products for ethical, allergic, or dietary reasons.
- Monosodium L-Glutamate (MSG) – Used as a stabilizer but sometimes linked to concerns about excitotoxicity in high doses (amounts in vaccines are minimal).
- Recombinant Human Albumin – Synthetic human protein used as a stabilizer; some individuals have ethical concerns about its use.
- Chick Embryo Cell Culture (Measles & Mumps Virus Growth Medium) – Derived from fertilized chicken eggs, which may be an issue for those avoiding animal products.*
*A note about egg allergies: the chick embryo cell culture used to propagate the measles and mumps viruses can be a potential concern for individuals with severe egg allergies. However, the amount of egg protein in the final vaccine is typically very low, and most people with egg allergies can safely receive these vaccines.
ProQuad (MMRV) and M-M-R II (MMR) are generally considered safe for individuals with egg allergies, and routine allergy testing is not required before administration. The yellow fever vaccine and some influenza vaccines, which contain higher amounts of egg protein, are more likely to cause reactions in people with severe egg allergies.
For individuals with a history of anaphylaxis to eggs, it’s always recommended to consult a physician before receiving any vaccine that involves chick embryo cell culture.
If you would like specific information regarding each ingredient, including studies, see: Vaccine Ingredients
Varicella FDA Package Inserts
- Varivax – MMR (Merck) Varivax FDA Package Insert – Refrigerator (no placebo-controlled trial was carried out with refrigerator-stable VARIVAX)
- Varivax (Merck) Varivax FDA Package Insert – Frozen
- ProQuad – MMRV (Merck) Measles, Mumps, Rubella, and Varicella combo. ProQuad FDA Package Insert* – Refrigerator
- ProQuad – MMRV (Merck) Measles, Mumps, Rubella, and Varicella combo. ProQuad FDA Package Insert* – Frozen
*From FDA Package Insert for ProQuad: Administration of ProQuad (dose 1) to children 12 to 23 months old who have not been previously vaccinated against measles, mumps, rubella, or varicella, nor had a history of the wild-type infections, is associated with higher rates of fever and febrile seizures at 5 to 12 days after vaccination when compared to children vaccinated with M-M-R® II and VARIVAX® (Varicella Only Vaccine) administered separately (8).
Controversy & Confusion About the Varicella Vaccine
Varicella Vaccine & Shedding
Because the varicella vaccine is a live virus vaccine, there is a theoretical possibility of viral shedding. However, this is something that is surveilled worldwide – and since 1995, only 11 healthy vaccinated people have spread the rash to 13 unvaccinated people. All these people had a rash after vaccination; therefore, if a rash occurs after vaccination, it is advised to keep it covered if possible and to stay away from vulnerable populations (9).
Varicella Vaccine & Pregnant Women
The varicella (chickenpox) vaccine is not recommended during pregnancy due to unknown effects on the fetus. However, the risk from the vaccine virus is expected to be lower than the risk from wild-type varicella infection which can have negative effects on the fetus as mentioned above. Here’s what you need to know if pregnant or planning to become pregnant:
Key Points (1):
- Do not get vaccinated while pregnant. The effects of the vaccine virus on fetal development are unknown.
- Wait at least one month after vaccination before becoming pregnant. This precaution helps minimize any theoretical risk.
- Accidental vaccination during pregnancy is not a reason to terminate. No cases of congenital varicella syndrome or increased birth defects have been linked to the vaccine.
- According to the CDC, pregnant household members are not a contraindication. If someone in the home is pregnant, others without immunity can still get vaccinated.
- However, if you’re pregnant and immune-compromised or have unknown varicella-immune status, check with your doctor before vaccinating a child in your household. It may be better to wait.
- No routine pregnancy testing is needed before vaccination. Doctors do not require a test before giving the vaccine to women of childbearing age.
- Report accidental vaccination during pregnancy. Merck and the CDC track cases through the Vaccine Adverse Event Reporting System (VAERS).
- Breastfeeding is safe after vaccination. Nursing parents can receive the varicella vaccine without concern.
If you are unsure about your immunity or have concerns about varicella exposure during pregnancy, talk to your healthcare provider.
Varicella Vaccine & MMR
The varicella vaccine is available on its own or in combination with MMR. They have interactions in terms of safety and efficacy that are important to know about.
There is a small but real increased risk of febrile seizure when giving MMRV compared to separate MMR and varicella vaccines. For children, and especially for those with a personal or family history of seizures, separating these vaccines is a wise idea. They can still be given in the same visit, simply at separate sites. If choosing to give at different visits, the varicella vaccine must be given at least 30 days after the MMR vaccine for optimal efficacy (1).
For children ages 5+ and adults without a personal or family history of seizure, it appears that the risk of MMR + varicella compared to MMRV is similar.
Varicella-Like Rash After Vaccination
Some children may develop a mild rash after getting the chickenpox vaccine. About 3% of children get a few spots near the injection site, while 4-6% develop a mild, chickenpox-like rash on other areas of the body. These rashes usually appear within 2-3 weeks after vaccination and tend to be small, with only a few spots. The rash may look like red bumps (maculopapular) rather than typical chickenpox blisters. In rare cases, the vaccine-related rash could be contagious, meaning it might spread the virus to others, especially people with weakened immune systems or those who have never had chickenpox (1). To be cautious, keep the rash covered and wash hands after touching it.
Varicella & Shingles Connection
Varicella (chickenpox) and shingles (herpes zoster) are caused by the same virus—the varicella-zoster virus (VZV). After a person recovers from chickenpox, the virus stays dormant in the nervous system, hiding in nerve cells near the spinal cord. For most people, the virus remains inactive, but years or even decades later, the virus can reactivate and cause shingles. This reactivation is more common in older adults, people with weakened immune systems, or those under significant stress. Shingles appears as a painful rash, often on one side of the body, and can lead to complications like long-term nerve pain (postherpetic neuralgia).
A logical question is, How does varicella vaccination in childhood affect shingles later in life? We don’t have definitive data just yet, but a 14-year study suggests that chickenpox vaccination in childhood results in lower incidence of shingles in those vaccinated later in life (10). However, it has also been suggested that the vaccination program could result in higher levels of shingles due to lower levels of immune boosting from varicella exposure in the community (11); so far, this has not been what is observed (12).
Though more research is needed, at present, it seems that the chickenpox vaccine reduces the change of shingles later in life.
Varicella Vaccine Reactions
Signs to Look for Include (13):
- Injection site reactions occur in 20% of kids and one-third of teens and adults.
- Fever is expected in 15% of people, and a mild chickenpox-like rash can occur in about 4% one to four weeks after the shot.
- Systemic reactions, such as body aches, general ill feeling, irritability, fatigue, intestinal symptoms, or headache, occur in about 85% of people after the first dose and 66% after the second.
Known Severe Reactions (13):
- Pneumonitis, a severe inflammatory reaction in the lungs occurs in 1:100 – 1:1,000 individuals.
- Seizures from fever side effect: 1:1,000.
Postmarketing Surveillance (13):
Adverse reactions that have been reported by the public since the introduction of the vaccine:
- Body as a Whole: Anaphylaxis (including anaphylactic shock) and related phenomena such as angioneurotic edema, facial edema, and peripheral edema.
- Eye Disorders: Necrotizing retinitis (in immunocompromised individuals).
- Hemic and Lymphatic System: Aplastic anemia; thrombocytopenia (including idiopathic thrombocytopenic purpura (ITP)).
- Infections and Infestations: Varicella (vaccine strain).
- Nervous/Psychiatric: Encephalitis; cerebrovascular accident; transverse myelitis; Guillain-Barré syndrome; Bell’s palsy; ataxia; non-febrile seizures; aseptic meningitis; dizziness; paresthesia.
- Respiratory: Pharyngitis; pneumonia/pneumonitis.
- Skin: Stevens-Johnson syndrome; erythema multiforme; Henoch-Schönlein purpura; secondary bacterial infections of skin and soft tissue, including impetigo and cellulitis; herpes zoster.
Who Should Not Get The Varicella Vaccine?
The varicella vaccine should not be given to individuals who (1):
- Have had a severe allergic reaction to a previous dose or a vaccine component (see above for a full ingredient list and/or check the up-to-date package inserts)
- Have a weakened immune system due to leukemia, lymphoma, generalized malignancy, immune deficiency diseases, or immunosuppressive therapy
- Have a family history of congenital or hereditary immunodeficiency in first-degree relatives
- Have HIV (varicella vaccine may or may not be recommended depending on CD4 count, but MMRV is strictly contraindicated)
- Have undergone a hematopoietic stem cell transplant (should wait at least 24 months)
- Are pregnant
Precautions to consider before getting the varicella vaccine:
- Moderate or severe acute illness* (wait until recovery)
- Alpha-gal allergy (consult with a physician)
- Recent receipt of antibody-containing blood products (wait 3 to 11 months)
- Need for tuberculosis testing (timing should be discussed with a healthcare provider)
- Use of specific antiviral drugs in the 24 hours before vaccination
- Simultaneous use of aspirin or aspirin-containing products due to the risk of Reye’s syndrome
- Personal or family history of seizures of any cause (consult with a physician – should get separate MMR and varicella vaccines rather than the MMRV)
*At Dr. Green Mom, we also prefer postponing vaccination in the case of mild illness, especially at the beginning of mild illness because it is unknown if the illness will become moderate to severe; mild remaining symptoms at the tail end of an illness are alright provided the child seems to have recovered in terms of energy levels.
The Bottom Line and the Varicella Vaccine
What is chickenpox? Chickenpox is a highly contagious virus that causes an itchy rash, fever, and fatigue. It spreads easily through coughing, sneezing, or direct contact with the rash. While chickenpox is usually mild in healthy children, it can cause complications like bacterial skin infections, pneumonia, or encephalitis (brain inflammation), especially in infants, pregnant women, and people with weakened immune systems. Once a person has had chickenpox, the virus stays in their body and can later reactivate as shingles.
How common is chickenpox in the United States? Chickenpox used to be extremely common, but because of the vaccine, cases have dropped by more than 90%. However, unvaccinated children and adults can still get chickenpox; breakthrough infections in vaccinated children and outbreaks sometimes happen in schools and childcare settings. Before the vaccine, almost everyone got chickenpox as a child. Now, most children are protected through vaccination or natural immunity from a past infection.
What are the risks of the chickenpox vaccine? The chickenpox vaccine has similar side effects to other vaccines, like soreness at the injection site, fever, and fatigue. Some children may develop a mild rash. Rare but serious risks include allergic reactions, seizures (caused by fever), and a very small risk of pneumonia or severe rash in people with weakened immune systems. Since this is a live virus vaccine, there is also a small chance of viral shedding; therefore, recently vaccinated people should avoid vulnerable individuals for six weeks after vaccination. Giving varicella separately from the MMR vaccine has a slightly lower risk of febrile seizure.
What are the benefits of the chickenpox vaccine? The chickenpox vaccine provides strong protection against severe chickenpox and its complications. Most vaccinated children who do get chickenpox have a much milder case with fewer spots, lower fever, and a quicker recovery. Two doses of the vaccine offer the best protection and reduce the risk of shingles later in life. The vaccine is especially important for children who are around newborns, pregnant women, or people with weakened immune systems, as it helps prevent spreading the virus to those who could have serious complications.
References:
- Centers for Disease Control and Prevention. (2021). Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, Kroger A, Wolfe S, eds. 14th ed. Washington D.C. Public Health Foundation. Chapter 22: Varicella. https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-22-varicella.html
- Klassen, T. P., Hartling, L., Wiebe, N., & Belseck, E. M. (2005). Acyclovir for treating varicella in otherwise healthy children and adolescents. The Cochrane database of systematic reviews, 2005(4), CD002980. https://doi.org/10.1002/14651858.CD002980.pub3
- Gnann Jr., J. W. (2007). Antiviral therapy of varicella-zoster virus infections. In: Arvin A, Campadelli-Fiume G, Mocarski E, et al., editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press. Chapter 65. Available from: https://www.ncbi.nlm.nih.gov/books/NBK47401/
- Levin, M. J., Duchon, J. M., Swamy, G. K., & Gershon, A. A. (2019). Varicella zoster immune globulin (VARIZIG) administration up to 10 days after varicella exposure in pregnant women, immunocompromised participants, and infants: Varicella outcomes and safety results from a large, open-label, expanded-access program. PloS one, 14(7), e0217749. https://doi.org/10.1371/journal.pone.0217749
- Centers for Disease Control and Prevention. (2021). About the Varicella Vaccines. Www.cdc.gov. https://www.cdc.gov/vaccines/vpd/varicella/hcp/about-vaccine.html
- Leung, J., Broder, K. R., & Marin, M. (2017). Severe varicella in persons vaccinated with varicella vaccine (breakthrough varicella): a systematic literature review. Expert review of vaccines, 16(4), 391–400. https://doi.org/10.1080/14760584.2017.1294069
- Zhu, S., Zeng, F., Xia, L., He, H., & Zhang, J. (2018). Incidence rate of breakthrough varicella observed in healthy children after 1 or 2 doses of varicella vaccine: Results from a meta-analysis. American journal of infection control, 46(1), e1–e7. https://doi.org/10.1016/j.ajic.2017.07.029
- Merck Sharp & Dohme LLC. (2024). ProQuad®. Product insert from the vaccine manufacturer. https://www.fda.gov/media/75195/download
- Centers for Disease Control and Prevention. (2024). Chickenpox (Varicella) Vaccine Safety. Www.cdc.gov. https://www.cdc.gov/vaccine-safety/vaccines/varicella.html
- Baxter, R., Ray, P., Tran, T. N., Black, S., Shinefield, H. R., Coplan, P. M., Lewis, E., Fireman, B., & Saddier, P. (2013). Long-term effectiveness of varicella vaccine: a 14-Year, prospective cohort study. Pediatrics, 131(5), e1389–e1396. https://doi.org/10.1542/peds.2012-3303
- Almutawa, Y. M. H. A. M., Bhattarai, E., & Zhao, J. J. (2024). Mechanism, impact, and effectiveness of herpes zoster vaccines: A comprehensive review. International Journal of Dermatology and Venereology, 7, E01-E30.
- Shaw, J., & Gershon, A. A. (2018). Varicella Virus Vaccination in the United States. Viral immunology, 31(2), 96–103. https://doi.org/10.1089/vim.2017.0136
- Merck Sharp & Dohme LLC. (2023). VARIVAX®. Product insert from the vaccine manufacturer. https://www.fda.gov/media/76000/download
Reviewed/Updated: 03/25
Content Created: 03/14